Proline
 Proline is a mysterious amino acid. With proline you basically should forget everything what you’ve learned about the amino acids, because this chap won’t fit in any common category.
Proline is a mysterious amino acid. With proline you basically should forget everything what you’ve learned about the amino acids, because this chap won’t fit in any common category.
On the one hand, the structure contains only 5 carbon atoms with no side chain functional group, looks easy, right? However, on the other hand, this is the only secondary amino acid in the canonical repertoire (sometimes people call it an imino acid, but this is wrong). The endocyclic amino-group restricts the set of allowed backbone conformations. In addition, peptidyl-prolyl linkages are tertiary amide bonds, prone to cis-trans isomerization issues. Let’s illustrate this argument with the help of a figure:
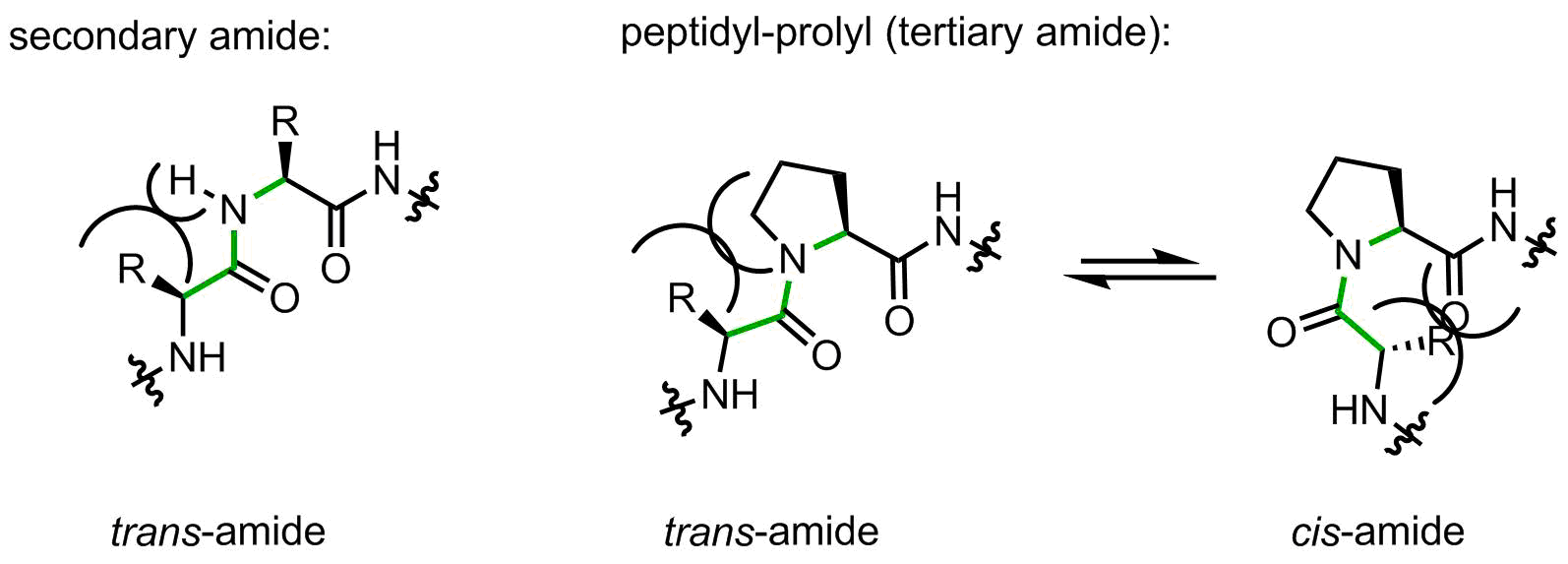
When a peptide bond is made by a secondary amide, the small substituent (hydrogen atom) is placed on the same side as the large upstream peptide fragment, thus making a trans-amide favorable. Indeed, in secondary amides the trans/cis preference is usually > 100:1.
Proline forms a tertiary amide. Now the upstream peptidyl moiety is placed next to the CH2-group, which is notably larger. The amide can flip to the cis-amide conformation with a little loss in energy. In peptidyl-prolyl fragments, the intrinsic preference towards trans-amide is reduced to 2-10:1 depending from the amino acid context, this means a very little intrinsic preference.
The flipping of the peptidyl-prolyl fragment from cis to trans conformation and back is well known in protein biophysics and biochemistry. Because the amide bond rotation is a slow process, this phenomenon can be considered as molecular timer in folding and in signaling. In nature, the correct positioning of some peptidyl-prolyl amides is facilitated by little helpers, chaperones called peptidyl-prolyl cis-trans isomerases.
Another famous feature of peptidyl-prolyl fragments is the lack of NH, which makes them unable to donate a backbone H-bond. This feature along with the backbone conformation restricted by the 5-membered ring make proline a breaker of common α-helices and β-sheets. The lack of NH make proline residues invisible for many biophysical methods based on NMR. The red-shifted light absorption profile of the tertiary amide also complicates UV-based conformational analyses, for example circular dichroism.
Oligomeric (Pro)n and proline-rich fragments forms a special type of fold called polyproline helix, with the polyproline-II helix forming preferentially. Ribosomal translation of proline into proteins is known to be a slow process for both, P- and A-sites. Sequences with 2-3 proline residues induce ribosome stalling, requiring a special elongation factor to alleviate the translation process (called elongation factor P, EF-P). In organic chemistry, proline’s famous property is its prominent organocatalytic activity in condensation reactions.
The classical dual hydrophobic/hydrophilic classification of amino acids fails completely when it comes to proline. Please, be aware that proline can be completely water exposed or be buried into a hydrophobic core of a protein. Both are possible depending from the context.
- Fischer, G. Chemical aspects of peptide bond isomerization. Chem. Soc. Rev., 29, 2000, 119-127, doi: 10.1039/A803742F
Classical review for the cis-trans isomerism around peptidyl-prolyl, mechanisms of the process and influence of the context amino acids.
- Kay, B. K. et al. The importance of being proline: the interaction of proline-rich motifs in signaling proteins with their cognate domains. FASEB J., 14, 2000, 231-241, doi: 10.1096/fasebj.14.2.231
This is an interesting discussion on how proline-rich fragments and polyproline conformation are involved in protein-protein interactions.
- Zondlo, N. Aromatic–Proline Interactions: Electronically Tunable CH/π Interactions. Acc. Chem. Res., 46, 2013, 1039-1049, doi: 10.1021/ar300087y
The paper illustrates molecular principles of the aromatic side chain – proline interactions using molecular models.
- Green, R. Getting Past Polyproline Pauses. Science, 339, 2013, 38-39, doi: 10.1126/science.1233338
Rachel Green explains the phenomenon of ribosome stalling on oligoproline stretches and how the ribosomes are rescued by EF-P. The discovery of oligoproline stalling was made independently by two groups from Göttingen (M. Rodnina) and Munich (K Jung, D. Wilson).
- Kubyshkin, V and Budisa, N. Amide rotation trajectories probed by symmetry. Org. Biomol. Chem., 15, 2017, 6764-6772, doi: 10.1039/C7OB01421J
Our recent molecular model, which illustrates how peptidyl-prolyl rotation should proceed.
- Dobitz, S. et al. Oligoprolines as Molecular Entities for Controlling Distance in Biological and Material Sciences. Acc. Chem. Res., 50, 2017, 2420-2428, doi: 10.1021/acs.accounts.7b00340
A recent review of the use of oligomeric proline sequences in material science and drug design applications.
>>> Genetic Code <<<
