Tryptophan
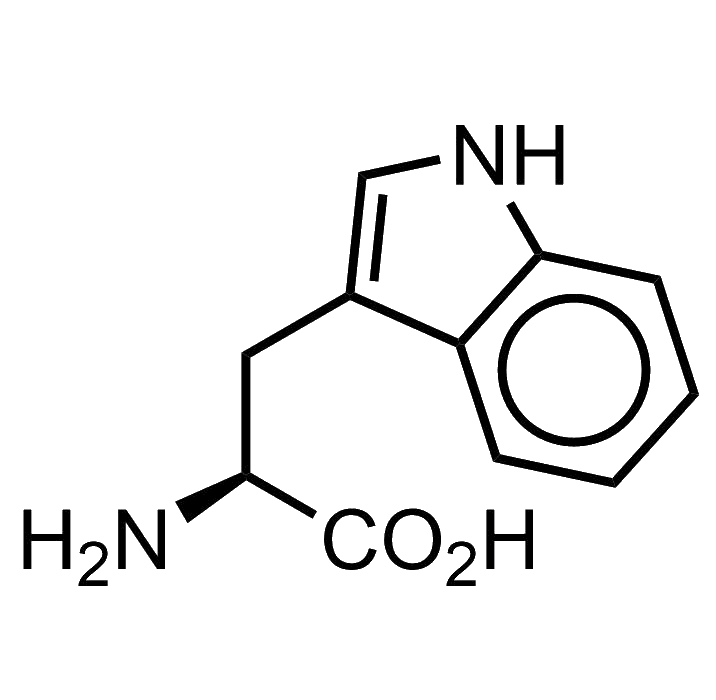 Tryptophan bears an indole as its side chain. This chemical moiety is a very versatile heterocycle, and there are many indole derivatives in biochemistry: secondary metabolites, antibiotics, toxins, drugs, you name it… LSD, well, yes, LSD too is an indole derivative.
Tryptophan bears an indole as its side chain. This chemical moiety is a very versatile heterocycle, and there are many indole derivatives in biochemistry: secondary metabolites, antibiotics, toxins, drugs, you name it… LSD, well, yes, LSD too is an indole derivative.
But what is the role of tryptophan when the amino acid lands into proteins? First, we can notice that indole is a large aromatic fragment, which can be hydrophobic. However, the presence the heterocyclic component creates a few dipole axes, and this creates a fairly decent overall dipolar moment of the molecule (2.2 Debye). The final outcome is that indole becomes amphiphilic, and therefore can occupy positions at interfaces between environments with different polarities. Classical example is the lipid membrane, where tryptophan often serves as an anchoring residue, with the indole penetrating between polar head-groups and fatty tails. Thus, in transmembrane helices tryptophan side chain would be responsible for correct positioning of the helix in the membrane.
There is another reason, why tryptophan residue becomes membrane-anchoring. If you look at the structure of the membrane, the lipid tail segment is fully packed with the fatty acid tails, while the head-groups are organized by various fragments like phosphacholine, phosphainositol, etc,, all these are also bulky. There is always a glycerol structure linking these two together, and this junction point becomes quite thin. As the result, the membrane structure forms a small region with underpressure at the tail/head-group interface. This vacuum section can easily suck in amino acid side chains with flat shape, such as aromatic structures. Since indole is also amphiphilic, this becomes an ideal place to harbor this side chain. Phenylalanine and tyrosine cannot overtake this job so easily, since these aromatics are too hydrophobic to be placed at an interface position. Tryptophan offers the best solution.
Let’s mention rich variety of post-translational modification chemistry with tryptophan, for example: hydroxylation, halogenation, geranylation, cyclization, etc. The main toxins of the red cap and death cap mushrooms, amanitins and phalloidins, have a cysteine-tryptophan side-chain-side-chain bridges, important for maintaining their evil bicyclic structures. In addition to the chemical versatility, tryptophan is extensively used in biochemical assays and biophysical studies due to its UV-absorption and fluorescence features.
Is tryptophan essential for life? Well… no. In 2015 we demonstrated that the function of tryptophan can be overtaken by thienopyrrole-alanine, and that the amino acid exchange to its analogue can be made globally in E. coli cells in all 21k positions in the proteome. Of course, E. coli was somehow an easy target, since there is no secondary metabolism or post-translational modifications with tryptophan in this organism. However, overall it demonstrates that on organism can live even when we exchange one amino acid by another, which is pretty spectacular.
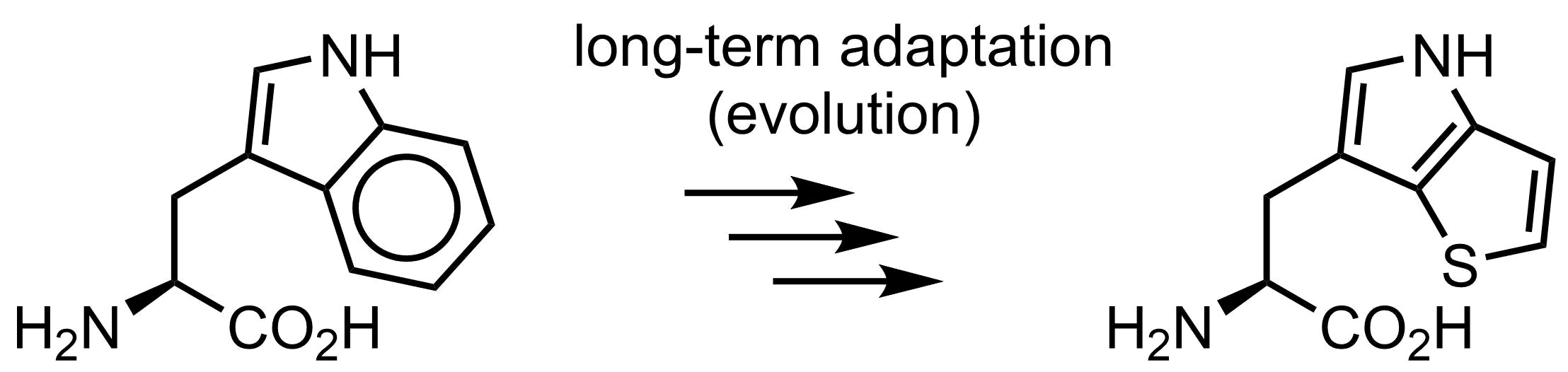
Why is then tryptophan structure in the genetic code? There should be reasons, and we in our lab have some discussions and ideas how this happened.
Interesting readings:
- Okada, M. et al. Posttranslational isoprenylation of tryptophan in bacteria. Beilstein J. Org. Chem., 13, 2017, 338-346, doi: 10.3762/bjoc.13.37
A recent review on post-translational isoprenylation of tryptophan, mainly coming from B. subtilis.
- Hoesl, M. G. et al. Chemical Evolution of a Bacterial Proteome. Angew. Chem. Int. Ed., 54, 2015, 10030-10034, doi: 10.1002/anie.201502868
Evolution experiment, where tryptophan was completely exchanged to thienopyrrolylalanine in E. coli.
- Matinkhoo, K. et al. Synthesis of the Death-Cap Mushroom Toxin α-Amanitin. J. Am. Chem. Soc., 140, 2018, 6513-6517, doi: 10.1021/jacs.7b12698
First chemical synthesis of the main toxin of the death cap mushroom. The molecule contains a side-chain macrocycle formed by Trp and Cys residues.
>>> Genetic Code <<<
